AliveCor’s EKG Monitoring Case For iPhone Gets FDA Approval.
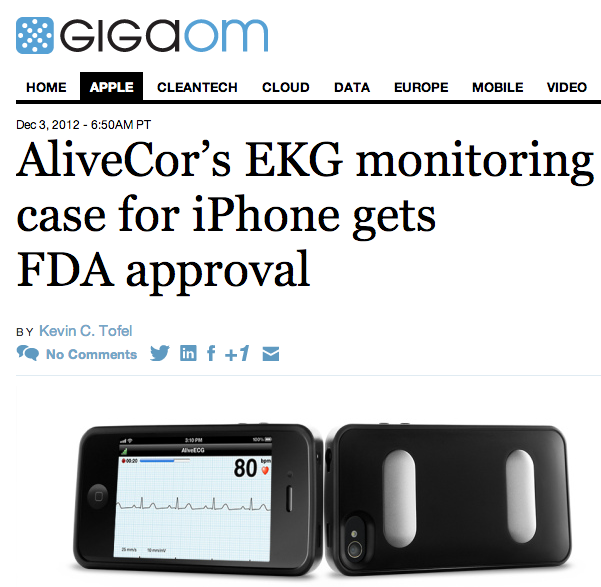
Don’t be surprised if your next doctor visit requires you to touch your physician’s smartphone: the U.S. Food and Drug Administration has approved an iPhone case from AliveCor that can monitor your pulse and heart rhythm. The $199 AliveCor Heart Monitor, which works with either an iPhone 4 or 4S, is available for pre-orders and should be shipping to medical professionals by January of 2013.
To use the device, patients place two fingers on the back of AliveCor’s iPhone case and rest them on the large electrodes. The case can also be pressed directly against a patient’s chest for ECG, or electro-cardigram monitoring, purposes. Data from the electrodes wirelessly passed from the case to the iPhone, where it can be analyzed or send to the cloud for storage. The case runs on its own coin-cell battery, which is good enough for an estimated 12,000 scans at 30 seconds in length.
While the case and supporting app won’t analyze the data — that’s for an actual medical professional to; at least for now — the idea of putting the data in the cloud is intriguing. A doctor could remotely view the data for analysis, for example. Or, if the device were actually sold directly to consumers, a doctor’s visit could be done virtually: The patient could use their own AliveCor case and iPhone to send heart rate data to a local physician at regular intervals.
Read the full article here.

